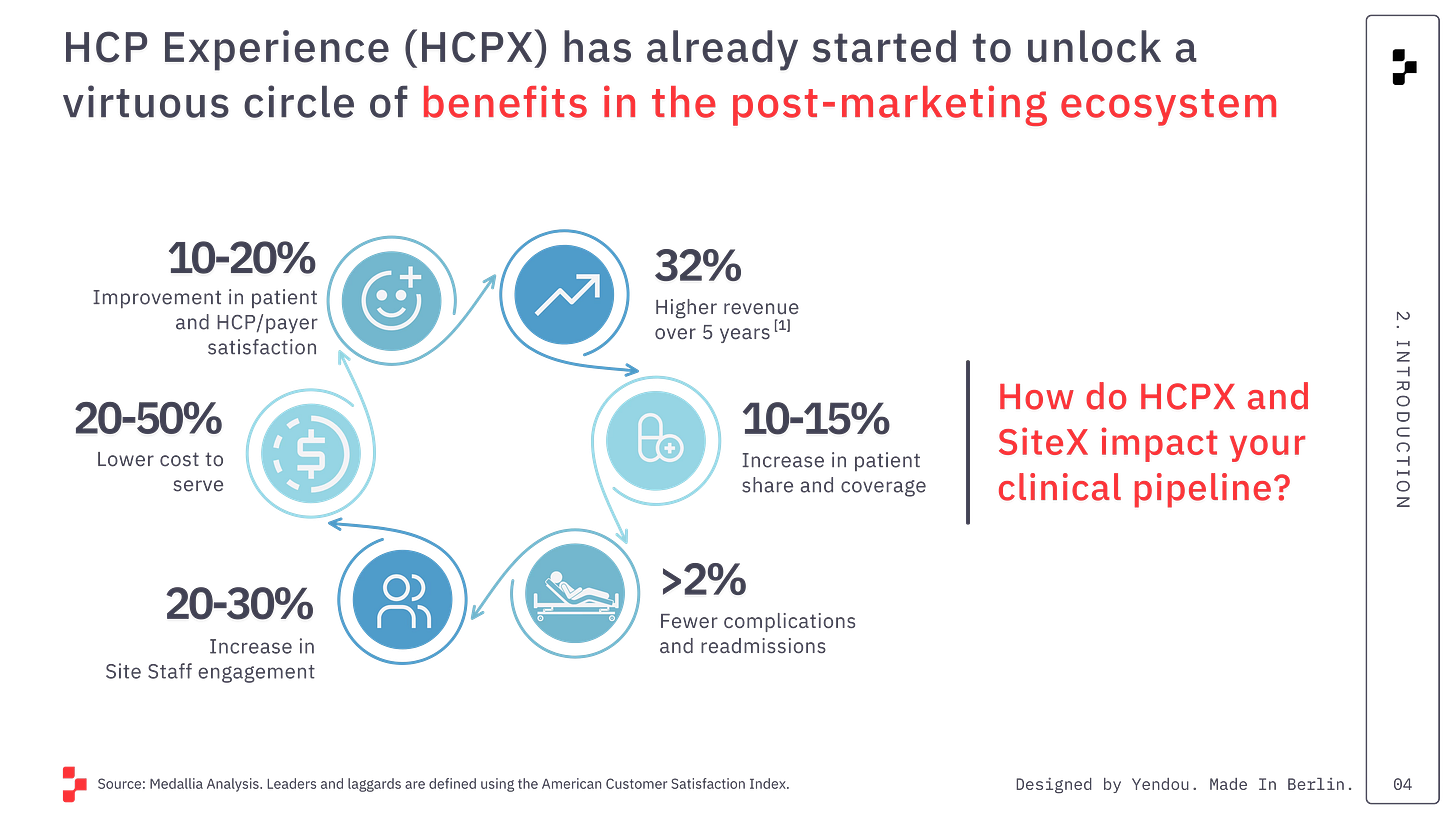
Why Commercial Teams Obsess Over HCP Data—And What ClinOps Must Learn
Why do we track HCP relationships obsessively in sales—but leave site engagement to spreadsheets and guesswork in R&D?
This is the question I want to address today.
In pharmaceutical R&D—where billions and years are spent chasing trial success—one overlooked truth cuts through the noise:
The most valuable data isn’t in your lab notebooks or regulatory filings. It’s in your relationships with research sites.
More precisely, it’s in how your team selects, engages, and contracts with these sites—the actual infrastructure powering your clinical pipeline.
And yet, while commercial teams have embraced this logic—investing in CRM platforms like Salesforce—clinical development lags far behind.
It’s not just a missed opportunity.
It’s a strategic vulnerability.
Salesforce, now worth $148 billion, was built on one idea:
“Businesses are inherently customer-centric, and the most valuable data is related to how they sell to and serve their customers.”
That mindset became the operating system for commercial teams across industries—including pharma.
Sales teams now use CRMs to track every touchpoint with HCPs and payers.
The result? A precision machine of customer insight, outreach optimization, and predictable revenue.
Now compare that with clinical operations.
Clinical development teams still operate without structured insight into their “customers”—the research sites that recruit patients, collect data, and run the studies.
Rather than building systems to capture and leverage this engagement, many companies outsource site selection and management to CROs, who then repackage the resulting data and sell it back as “intelligence.”
Others rely on EDC vendors whose business models thrive on locking up insights.
Or worse—internal teams manage site relationships through scattered emails, PDFs, Excel sheets, and unstructured calls that no one records, centralizes, or learns from.
The result?
The most valuable data in clinical operations—how teams select, budget, contract, and engage with sites—is never captured systematically.
This creates a broken feedback loop:
No memory. No pattern recognition. No learning.
And no path to becoming a site’s partner of choice.
This oversight comes at a steep cost.
Site performance—activation timelines, enrollment speed, retention rates, and data quality—directly dictates trial timelines and economics.
Feasibility and study start-up alone can eat up months and millions in lost opportunity.
Without a system to manage and learn from these relationships, teams lose their edge—and pipelines become vulnerable to delays, inefficiencies, and competitors who move faster.
Sales organizations internalized what Salesforce knew: customer data is the company’s lifeblood. For clinical teams, the parallel is clear—the most valuable data lies in how they manage their site relationships.
So why hasn’t the industry built an SRM—a Site Relationship Management Platform—to mirror this logic?
A system of record that captures how an organization works with research sites and feeds that knowledge back to accelerate execution could transform clinical development. Data has gravity. It commands value. And it pulls every connected system into orbit.
But a system of record alone isn’t enough.
The future lies beyond traditional CRMs that just track records and milestones.
What’s coming is Customer Engagement Management (CEM)—a new generation of platforms designed to become systems of intelligence.
What are systems of intelligence?
I’ll go deeper into this in a follow-up post next week.But here’s a quick definition.
For pharma and biotech, a CEM could revolutionize site engagement by replacing, automating, or redesigning entire workflows—generating new value from the intelligence captured during site interactions.
Imagine systems that personalize workflows based on a team’s portfolio, employee type, and the unique dynamics of site engagement. This isn’t theory. It’s happening now.
If site relationships dictate pipeline success, why aren’t we investing in the tools to master them?
The answer could unlock faster trials—and a reimagined R&D paradigm.
The future of clinical trials won’t be decided in the lab. It will be won in the relationships we choose to track—or ignore.
Announcement!
Are you leading Feasibility, Study Startup, Clinical Operations, or Clinical Development? The digital backbone of your work is being rewritten. And This is your chance to shape it.
Yendou invites senior R&D leaders to join its Early Adopter Advisory Board. As a design partner, your insights will shape how innovation reaches patients—faster, smarter, and globally.