The White Space Problem: Why Clinical Trials Still Take 16+ Months to Start
Today, I’d love to talk about the time gap between protocol design and the moment a clinician can enroll a patient in a clinical trial, also known as the Site Identification to Site Initiation Visit, or the feasibility and study start-up phase.
We call it the white space.
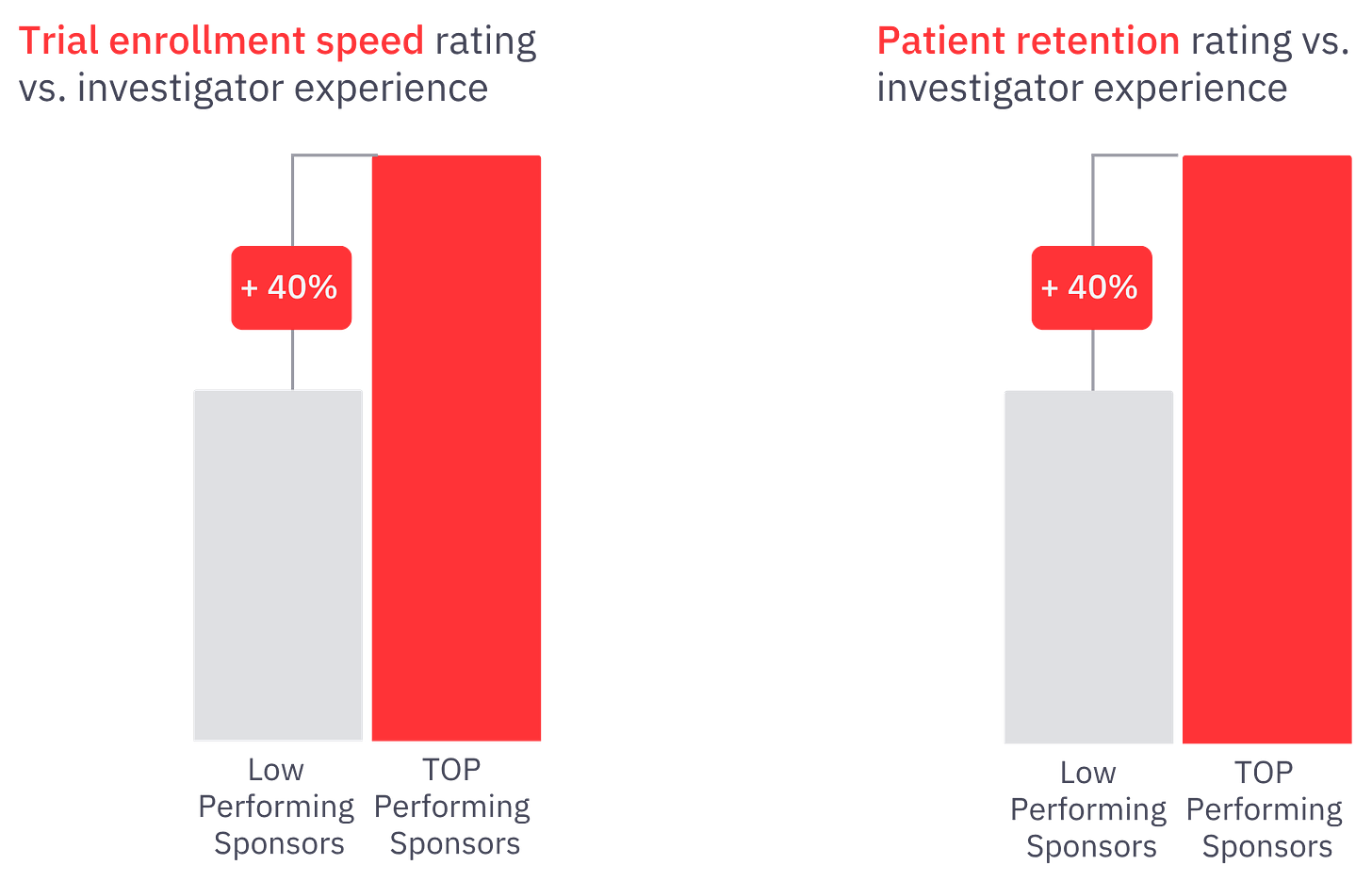
According to IQVIA and Thermo Fisher data (see caption), this phase now accounts for 25% of timelines in oncology and over 40% in cardiovascular trials.

At Yendou, we’ve made it our mission to collapse this white space. And we discovered that the main barrier is poor site engagement.
Why is Site Engagement the issue impacting current timelines?
To initiate a clinical trial, pharma/BioTech and CRO companies must:
1. Design a protocol in collaboration with key opinion leaders to answer a scientific question regarding the efficacy of a new innovation compared to the standard of care (Protocol Design).
2. Collect data on site and investigator capabilities to decide at which sites the clinical trial should take place (site selection).
3. Collect essential documents to gain permits to run the clinical trial at the selected sites (regulatory submission).
4. Enter into commercial agreements with sites to initiate the collaboration (CTA).
5. Once these steps are completed, initiate protocol, staff, and vendor training to ensure sites can perform the protocol well (site training and activation).
Each of these steps requires multiple interactions with sites. And by the time it’s all done… well, a lot of time has passed.
Site Engagement: The Challenges of Scale
For organizations that run multiple clinical trials annually, the Site identification to Site initiation process is repeated for every trial because data collected across teams and studies is not centralized.
A trial that could start in 6 months now takes 12 to 16 months because the site information necessary to make site selection decisions, and essential documents necessary to execute IRC submission has to be recollected. Again. And again.
Understaffed healthcare systems aggravate this issue, as sites are often slow to respond, resulting in lengthy waiting periods for ClinOps teams to gather the necessary documents and data.
This delay:
Prolongs trial initiation and drug approval timelines.
Increases operational costs.
Risk reducing Pharma & BioTech market coverage, especially for highly competitive indications.
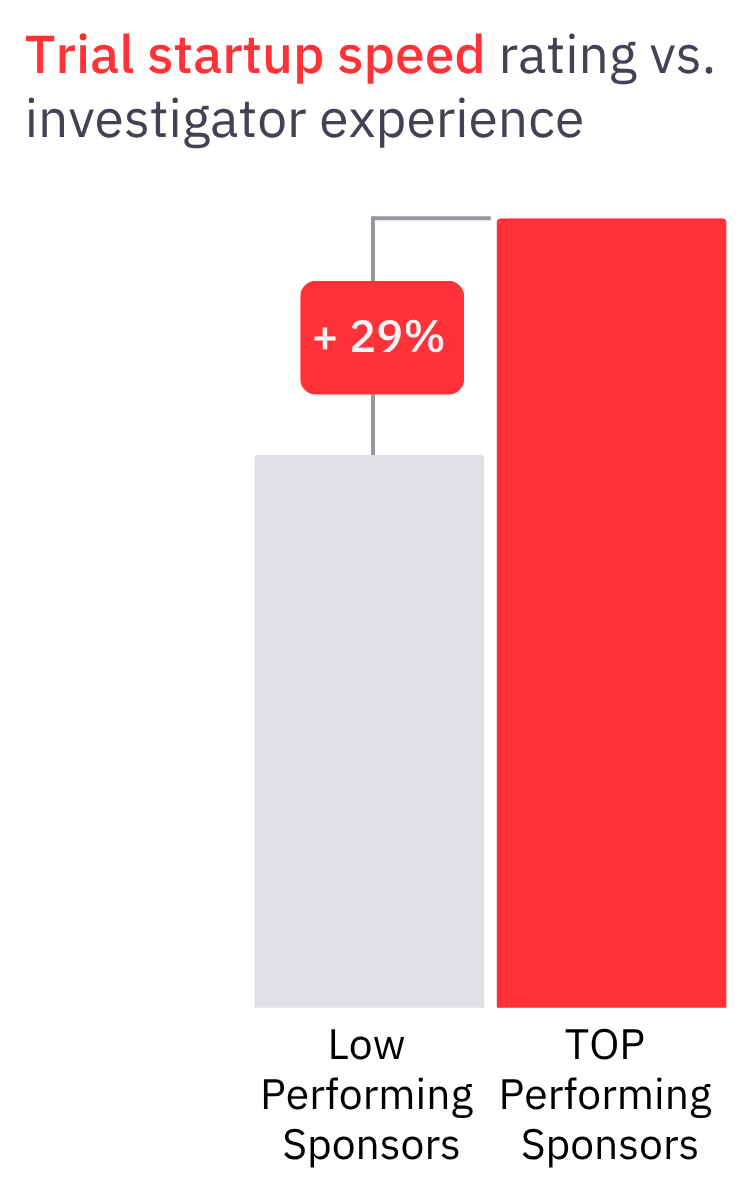
Companies respond to these challenges by pressuring ClinOps teams to aggressively push sites for faster performance. This negative site engagement experience, harms the sponsor-site relationship and instead of enhancing the performance, it diminishes sites responsiveness leading to study startup timelines delay of up to 29%.
Beyond operational inefficiencies, ClinOps teams report dissatisfaction with their work. They spend much of their time - up to 60% -on repetitive administrative tasks, collecting data they have already gathered, instead of focusing on strategic initiatives or relationship development.
Here the story of a PhIII TNBC trial feasibility journey (one of many, that many of us can relate to):
Site Engagement: Investigators’ Dissatisfaction
On the other side, we frequently receive complaints from investigators about their dissatisfaction with current sponsor-site relationships.
To them, life sciences companies don’t know them well.
Instead of engaging in scientific and clinically driven conversations that honor their expertise and enable them to play an active role in developing protocols that impact patient journeys and improve the odds of a trial’s success, they feel the conversations are mechanical, process-oriented, and focused on infrastructure and auditing activities.
When we talk to investigators and clinical operations teams, the recurring issues are clear:
Repetitive Data Collection: Sites are frustrated by the constant need to provide the same information repeatedly.
Poor Investigator Engagement: Investigators feel undervalued and unheard. They say they often meet sponsors who don’t even remember their prior collaboration, request information and ghost them, etc…
Sites as Infrastructure and Patients Providers, Not Scientific Partners: Sites feel treated as mere infrastructure providers, there to offer patients and data, rather than respected subject matter experts.
The perception stems from how trials are conducted. Often, sites and investigator engagement within the clinical trial setting focuses only on infrastructure, auditing, and paperwork, ignoring the human element and expertise sites bring to patient care.
As one investigator said,
“It takes away all the excitement about the innovation.”
Commercially driven investigators perceive the CTA process as unpleasant in most oncology trials, citing it as a primary factor that impacts their dedication to patient recruitment.
It turns out that poor site identification-to-initiation experiences significantly impact clinical trial success and timelines.
The Bigger Question
If the problem is just the experience, how did we end up unintentionally creating a destructive cycle of collaboration that sabotages the industry and scientific progress?
When analyzing the 16-month site identification-to-initiation experience for both sponsors, CROs (referred to as life sciences companies), and research site teams, we came to the realization that all issues stem from one source:
Life sciences companies don’t know their sites well. And sites resent that.
Due to the lack of data gathered on companies’ site portfolio, ClinOps teams cannot deepen their relationships over time. Instead, the sponsor-site relationship remains superficial, limited to “qualification, auditing, and monitoring.”
It’s like going on multiple dates with someone you really like, but every time you meet them, they start the conversation with: “I’m very sorry, what was your name again?”
This frustrates everyone—teams at CROs, sponsors, and sites. Over time, the relationship becomes transactional, as everyone gives up on having meaningful relationships with the other party. By meaningful relationships, I mean science-driven, effective, and productive conversations that advance science both in terms of innovation and approval timelines.
The Fix? Don’t Automate Dysfunction. Replace It.
To us at Yendou, the only way to fix site identification-to-initiation timelines is by fixing sponsor-site engagement. Better engagement equals better collaboration and faster timelines.
After interviewing over 300 feasibility and startup leaders across Pharma, CROs, SMOs and research sites, we identified five capabilities sponsors must build:
Knowledge Centralization – A single source of truth for every site interaction.
Knowledge Retention – So information survives beyond a single study or team member.
Knowledge Collection Automation – Eliminate bottlenecks like CDA collection and feasibility form follow-up.
Internal Site Intelligence – Know your best-performing sites through your own data, not vendor metadata.
Engagement Quality Tracking – Measure responsiveness, readiness, and site experience over time.
Each of these capabilities moves Site Identification to Site Initiation Visit milestones from a process to a byproduct of knowing your site network.
Why This Matters
Fixing feasibility and study-startup workflows won’t fix feasibility and study-startup delays. Only deep, continuous, intelligent site engagement will.
This was Trials & Triumphs - On the relentless pursuit of progress at the intersection of scientific breakthroughs and operational reality.
Enjoyed the read? Share it. Spark new thinking.
Sincerely,
Zina